P1 – Chemistry at the Crack Tip
 Motivation
Motivation
The chemical environment can critically affect the fracture processes, leading to subcritical crack growth [1], [2], [3]. The inner surfaces of the cracks are covered by adsorbates from the surrounding liquid or gas phase. When bonds break in the course of crack propagation, these adsorbates strongly react with the newly created surfaces, for example, by saturating the broken bonds. Examples are stress corrosion cracking in metals and semiconductors or the moisture-driven crack growth in silica. In both cases, the crack propagation induces and drives the incorporation of oxygen species, leading to an oxidation/hydroxylation of the inner surfaces, which completely alters the chemistry at the crack tip [3]
Objectives
In this project we propose to study the complex interplay between bond breaking at the crack tip and the adsorption/bond saturation with molecules from the environment by MD simulations. The aim is to obtain mechanistic insights into environmentally-assisted fracture for model ceramic materials [4].
Work plan
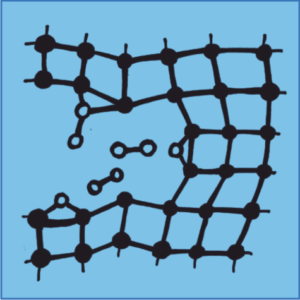
For an unbiased description of breaking and forming chemical bonds we will use quantum-chemical methods, specifically, density-functional theory (DFT) and, at a later stage, semiempirical DFT-based tight-binding (TB) models, which we derive by a systematic coarse-graining of the DFT electronic structure [5], [6]. The crack propagation will be modelled using a small representative cut-out of a sub-critically-loaded crack tip with fixed (according to boundary conditions given by the applied stress field, Erik Bitzek) and saturated outer atoms (see [7] for a typical setup). MD simulations of chemical processes during crack propagation will be done first for constant particle numbers while re-evaluating the adsorbate-coverage of the crack flanks after regular intervals by the thermodynamic surface phase diagram technique [8], [9], [10]. Later, MD simulations at a constant chemical potential will be performed by using a grand canonical MD technique, recently developed by us, in which molecules are exchanged on the fly between a reservoir and the inner crack surfaces depending on the stability of the adsorbate structure. Chemical reaction steps during crack propagation will be accelerated by metadynamics [11].
[1] T. L. Anderson, Fracture Mechanics, Boca Raton: CRC Press, 2005.
[2] R. P. Wei, Fracture Mechanics, Cambridge: Cambridge University Press, 2010.
[3] A. Gleizer, G. Peralta, J. R. Kermode, A. De Vita and D. Sherman, “Dissociative Chemisorption of O2 Inducing Stress Corrosion Cracking in Silicon Crystals,” Physical Review Letters, vol. 112, 115501, 2014.
[4] S. M. Wiederhorn, E. R. Fuller and R. Thomson, “Micromechanisms of crack growth in ceramics and glasses in corrosive environments,” Metal Science, vol. 14, no. 8-9, pp. 450-458, 1980.
[5] A. Urban, M. Reese, M. Mrovec, C. Elsässer and B. Meyer, “Parameterization of tight-binding models from density functional theory calculations,” Physical Review B, vol. 84, 155119, 2011.
[6] J. T. Margraf, M. Hennemann, B. Meyer and T. Clark, “EMPIRE: a highly parallel semiempirical molecular orbital program: 2: periodic boundary conditions,” Journal of Molecular Modeling, vol. 21, 144, 2015.
[7] J. R. Kermode, T. Albaret, D. Sherman, N. Bernstein, P. Gumbsch, M. C. Payne, G. Csányi and A. De Vita, “Low-speed fracture instabilities in a brittle crystal,” >Nature, vol. 455, no. 7217, pp. 1224-1227, 2008.
[8] B. Meyer, “First-principles study of the polar O-terminated ZnO surface in thermodynamic equilibrium with oxygen and hydrogen,” Physical Review B, vol. 69, 045416, 2004.
[9] P. M. Kowalski, B. Meyer and D. Marx, “Composition, structure, and stability of the rutile TiO2 (110) surface: Oxygen depletion, hydroxylation, hydrogen migration, and water adsorption,” Physical Review B, <vol. 79, 115410, 2009.
[10] L. Martínez-Suárez, J. Frenzel, D. Marx and B. Meyer, “Tuning the Reactivity of a Cu/ZnO Nanocatalyst via Gas Phase Pressure,” Physical Review Letters, </vol. 110, 086108, 2013.
[11] J. Kiss, J. Frenzel, N. N. Nair, B. Meyer and D. Marx, “Methanol synthesis on ZnO(0001). III. Free energy landscapes, reaction pathways, and mechanistic insights,” The Journal of Chemical Physics, vol. 134, 064710, 2011.
